

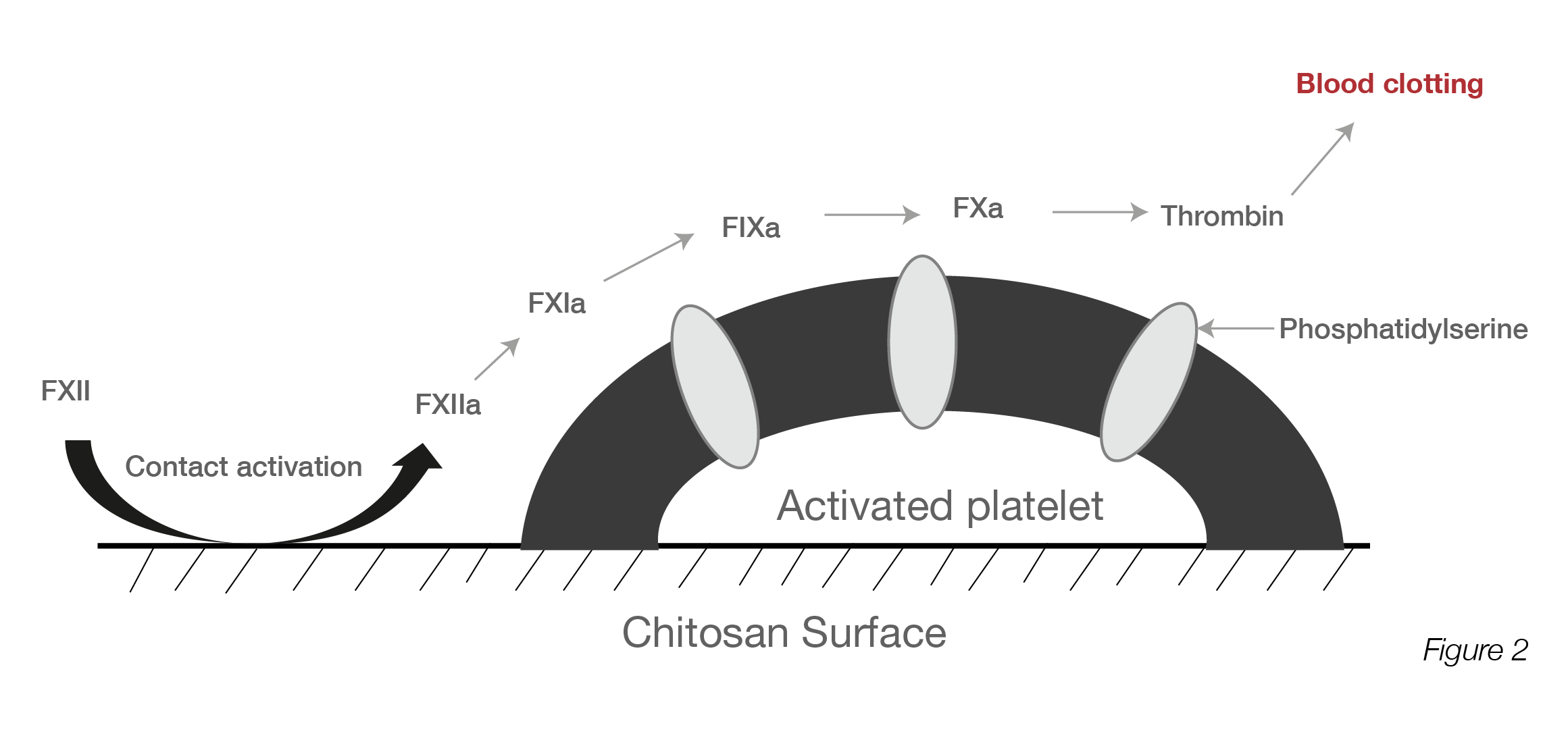

Figure 1: SEM images of platelets adhered on the chitosan dressing: a) Chi-B, (b) Chi-E-90, (c) Chi-E-80, (d) Chi-E-70, and (e) Chi-E-60. m Quantification of platelet adhesion on the chitosan films. Notes: n ¼ 4, *p < 0.001 relative to the Chi-B, # p < 0.01 relative to the Chi-E-80, # # p < 0.001 relative to the Chi-E-80 (Reproduced from Reference #2- He et al., 2013)
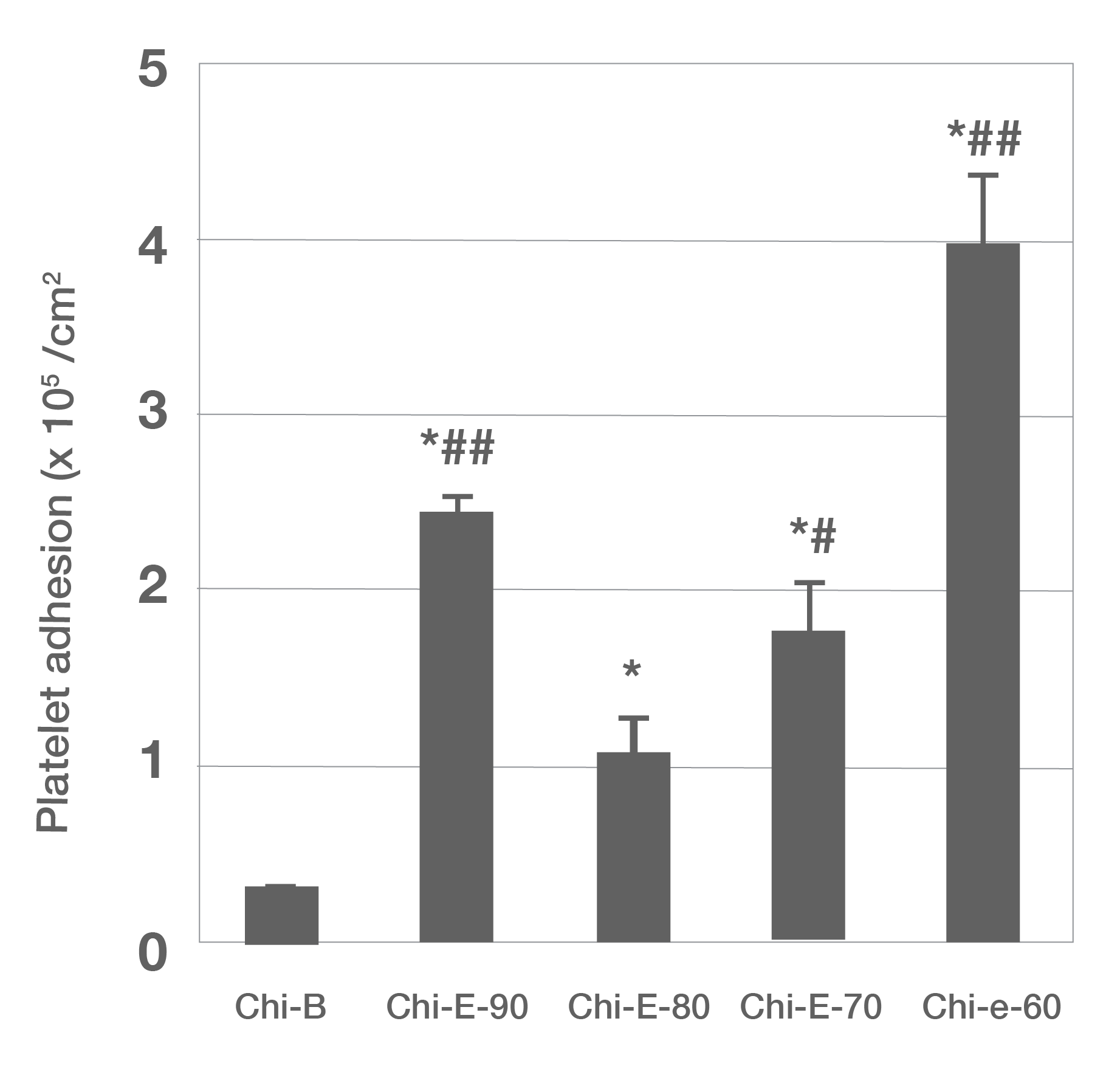
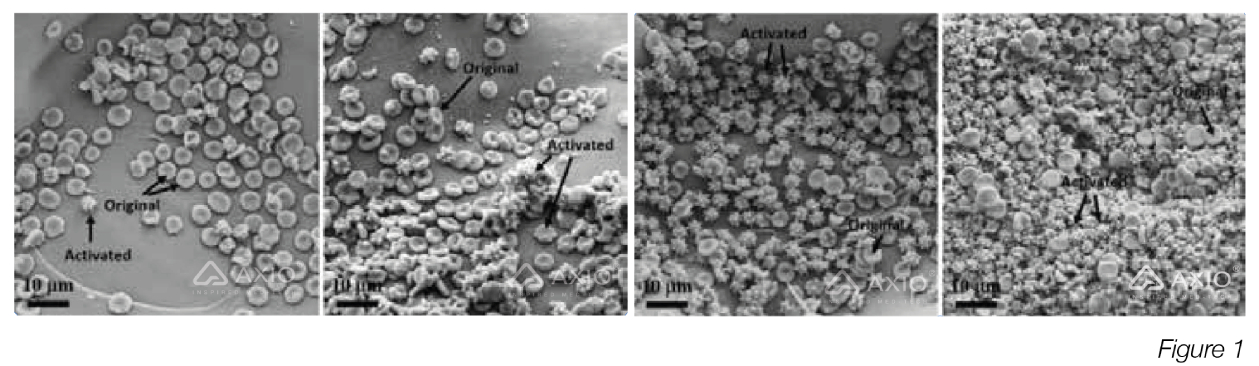
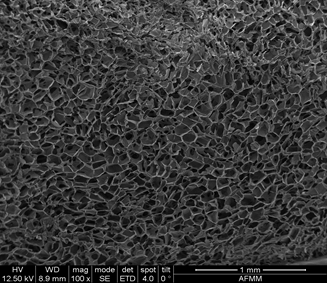
Figure 1: SEM images of erythrocyte coagulation on chitosan (A), CS 6c (BJ, CS 12c (C), and CS 1 Bc (D) (Reproduced from Reference #2- Chen et al.,2017)

Speak to the team
Our office is open Monday - Friday 8.30am - 5.00pm
Feel free to get in touch direct or leave us a message on the contact form and the team will get back to you
Phone
+44 (0) 1934 53 34 53
Address
Unit 6 Cambridge Mills, Dursley Road, Cambridge, Gloucestershire GL2 7AA
